12 Questions to Help Guide Renal Impairment PK Study Design
By Sabina Paglialunga, PhD Director Scientific Affairs, Celerion
Need to run a renal impairment pharmacokinetic (PK) study, but don’t know where to begin? We have you covered! Celerion has managed more than 30 renal impairment PK studies over the past decade. We have a network of clinical sites and access to patients. To begin, we’ll guide you through 12 key questions to optimize study design.
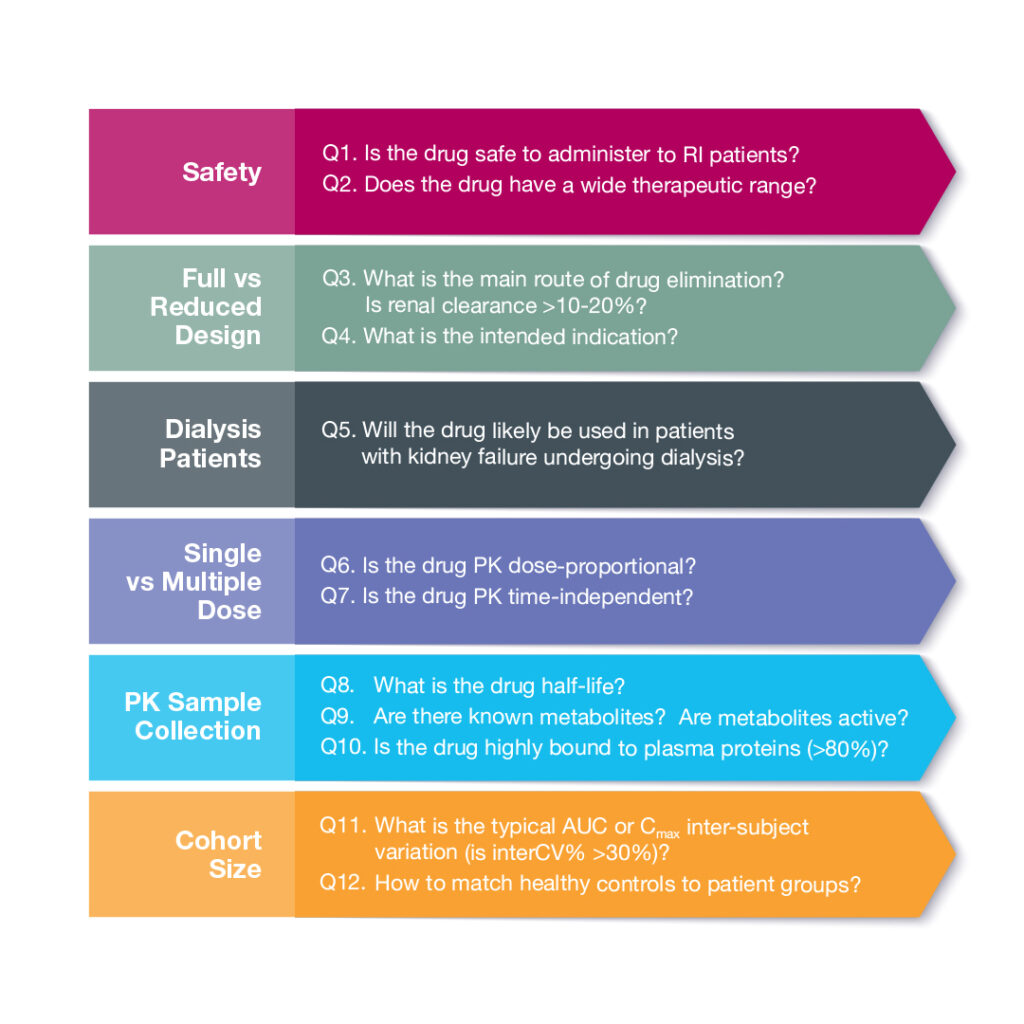
Safety First
The first set of questions (Q1&2) relates to the safety profile of the drug in development. Is the investigational product safe to administer to patients with kidney dysfunction? If the study drug exacerbates kidney dysfunction, then it may not be suitable to dose in renal impaired participants. Next, we should consider the therapeutic range, as an increase in adverse events (AEs) and safety concerns may arise in patients with altered kidney function if the range is narrow.
Design Foundations
Assuming we are in the clear on these two fronts, we can then address the type of study needed (Q3&4). The FDA guidance, refers to full and reduced study designs. A full study explores the spectrum of organ dysfunction with cohorts ranging from normal → mild → moderate → severe, and in some cases kidney failure. A reduced study examines both ends of the continuum to compare normal vs severe conditions. If the investigational drug is mainly eliminated via the kidneys (i.e. renal clearance is > 10-20%) and/or is intended for patients with chronic kidney disease (CKD), then a full study is typically recommended. A reduced design is often sought when the investigational drug is likely to be administered to CKD patients yet the study drug is predominantly eliminated via the hepatobiliary route.
A hemodialysis study (Q5) may be recommended if the study drug is likely to be used in patients undergoing dialysis; the drug is not highly bound to plasma protein, and is small enough to escape dialysis filtering. In this case, the PK of the drug and any active metabolites are evaluated in patients both on- and off-dialysis days.
The dose regimen will depend on the PK characteristics of the study drug (Q6&7). A single dose may be administered if the drug exhibits dose-proportionality and displays time-independent PK. Multiple dose administration is often recommended when the drug or active metabolites show dose- or time-dependent PK characteristics.
Sample Collection
The following series of questions (Q8-10) help define the study sample schedule, collection, and analysis. In general, for renal impairment PK studies, blood samples are collected out to at least 3x the drug half-life. Metabolites representing >10% of total drug concentration should be measured in addition to the parent drug. If there is significant plasma protein binding, the unbound drug concentration should also be analyzed. Plasma protein binding is often altered in patients with renal impairment. Per the FDA guidance, a limited number of unbound samples can be measured if binding is not concentration- or time-dependent. In this situation, we recommend to collect at least 2 time points per participant; one at baseline and one at Tmax.
Cohort Size
The next question to consider is the number of patients per cohort (Q11). The FDA guidance recommends a powered study and sample size justification based on PK variability. Depending on drug variability (interCV%), 9-14 patients per cohort may be recommended.
Normal control matching is another question that regularly comes up during renal impairment study design discussions (Q12). We typically recommend the healthy control group to match patients by age (± 10 years), BMI (± 20%) and gender. There are two strategies, individual and mean matching. Individual or 1-to-1 matching allows for parallel patient and control enrollment, but can be difficult if a patient has uncommon characteristic. In some cases, a healthy control can match to more than one patient from different disease stage cohorts. With mean matching, the average values for the patient group are matched to the control subjects. This approach requires fewer subjects, but must wait until patient enrollment is complete.
Ready to Start
With this information in hand, our team of operational and scientific experts can propose the ideal number of sites, recruitment timelines, and other study design considerations. Rely on Celerion for budget-friendly and streamlined processes that leverage our long-standing and established relationships with key renal impairment PK Principal Investigators.
Check out our Renal/Hepatic Impairment PK Study Resource page for more information, or contact us at info@celerion.com
